 Written by: Heather Clemenceau
Written by: Heather Clemenceau
The most recent news surfacing today is that Nestlé, one of the largest food companies in the world, has now been entangled in the EU horsemeat scandal. Major supermarket chains Tesco and Aldi were found to be selling beef products that contained horse meat. Burger King sourced thousands of burgers from the same Irish beef supplier, Silvercrest, and Findus “beef” lasagna was found to contain 100% horse meat.
Liffey Meats in Ireland and Dalepak in Yorkshire have both been fingered as well. Silvercrest and Dalepak are both subsidiaries of ABP Food Group, one of the largest beef processors in Europe. Huge blocks of frozen meat in cold storage in Northern Ireland – Freeza Foods, which had been quarantined by officials suspicious of its labelling and state of packaging, were found to contain 80% horse.
There is now evidence that both Polish and Italian mafia gangs are running multimillion-dollar scams to substitute horsemeat for beef during food production. There are claims that vets and other officials working within slaughterhouses and food production plants are intimidated into signing off meat as beef when it is in fact cheaper alternatives such as pork or horse.
 What this multi-level fraud has done is remove informed consent from the public – people believed they were paying for and consuming beef products. The public also had no idea that they were at risk for consuming minute quantities of veterinary drug phenylbutazone(“bute”) as a result of massive quantities of horsemeat of unknown origin entering the food chain.
What this multi-level fraud has done is remove informed consent from the public – people believed they were paying for and consuming beef products. The public also had no idea that they were at risk for consuming minute quantities of veterinary drug phenylbutazone(“bute”) as a result of massive quantities of horsemeat of unknown origin entering the food chain.
The Department for Environment, Food and Rural Affairs (Defra) has announced that there are at least eight known cases of horse carcasses that tested positive for phenylbutazone in the EU out of 206 samples. British Environment Minister David Heath told the House of Commons that of these eight horses, “three may have entered the food chain in France. The remaining five have not gone into the food chain.”
Phenylbutazone or “bute” was at one time marketed for humans use under the trade name of butazolidin. It was a Non-Steroidal Anti-Inflammatory (NSAID) used for arthritis and other inflammatory ailments that worked by inhibiting an
enzyme that synthesizes chemical mediators called prostaglandins. It was ultimately withdrawn by the FDA for causing a wide range of serious side-effects including blood dyscrasias which damage the bone marrow. It remains however, on the market for treatment for horses and is an effective anti-inflammatory. It is also prohibited in the food chain as residues of bute and its metabolite, oxyphenbutazone are not known to have safe limits.
Horse welfare advocates and the inadvertent consumers of horse meat have been repeatedly reassured by various government agencies and horse slaughter proponents that any residues of bute or its metabolite are harmless. We know that the pro-slaughters aren’t relying on science when they tell us this, but what about government agencies? On what do they base this reassurance?
“It is important to note that the levels of Bute in horsemeat, even if it is found, will be very low, and greatly below the doses following medical treatment in people that have been associated with occasional rare adverse reactions; therefore whilst this is unacceptable the actual risk to consumers is very small.”
“The main toxicity concern in humans is that some people developed (very rarely – 1 in 30,000 to 1 in 50,000 persons) an anaemia which was life threatening, when the drug was used clinically in humans. This occurred when the drug was used therapeutically in humans at a dose rate of some 2 to 6 mg/kg, similar to the current dose for the horse of 4.4 mg/kg.”
The question is whether the presence of bute in horsemeat can present a risk to human health even in small amounts. In the above noted tests, the highest amount of bute found in a horse carcass was 1.9 mg. If a human had been taking butazolidin in the 50s, they might have taken 200-400 mg a day in total, if we compare it to the current-day dosage of Tylenol or Advil. Obviously, we would have to consume a significant amount of contaminated horsemeat in order to reach the level of a therapeutic drug dosage.
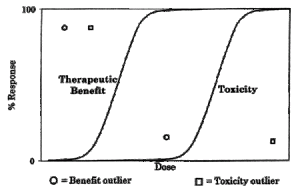 What is not clear, despite reassurances, is the level that is necessary for the average person to consume in order to experience a toxic effect. The basis for determining toxicity levels to inform public policy decisions has been the dose-response relationship, which is central to defining “safe” and “hazardous” levels and dosages for drugs, potential pollutants, and other substances to which humans are exposed.
What is not clear, despite reassurances, is the level that is necessary for the average person to consume in order to experience a toxic effect. The basis for determining toxicity levels to inform public policy decisions has been the dose-response relationship, which is central to defining “safe” and “hazardous” levels and dosages for drugs, potential pollutants, and other substances to which humans are exposed.
If a therapeutic does of butazolidin was once considered “safe” at 200-400 mg, then how do we know that some individuals are safe at 1.9 mg? If butazolidin was withdrawn from the market as being unsafe for some people at that dosage, we don’t know whether sensitive individuals may have experienced toxicity at lower levels as well. What about drug interactions? There is an acknowledged interaction between phenylbutazone and the anticoagulant drug warfarin, and patients taking warfarin can suffer severe gastrointestinal bleeding if they also take phenylbutazone. This complicates arguments about safety of bute in horsemeat. Bute also metabolizes to oxyphenbutazone, which has been shown to have similar toxicity. Have any of these horse carcasses been tested for oxyphenbutazone? Both bute and oxyphenbutazone bind to human serum albumin (HSA) as does warfarin and so they “compete” with each other. For more information on this interaction, please read this study by the Department of Pharmacology at the University of Western Australia.
If it still seems as though a negligible trace of bute in meat might not be enough to cause harm, there is an analogous cautionary tale of another NSAID – diclofenac, which was also used in human medicine for decades, and was recently introduced for veterinary use in India. Obviously, the dynamics are not the same, but vultures appear to have been exposed to the drug while scavenging livestock carcasses, their main food source, and this has accounted for death by renal failure of many vultures examined in a three-year study by the scientific journal Nature. Further investigation showed that diclofenac was fatal to vultures at 10 percent of the recommended dose. Tissue residues in livestock treated at the labelled dose rate were sufficient to cause death in vultures. These findings confirmed that diclofenac is the primary cause of the Asian vulture decline.
“As few as one in 760 carcasses containing diclofenac at a dose lethal to vultures would be sufficient to cause the observed decline in vulture numbers (30% per year). Clearly, even small-scale usage of the drug can have catastrophic consequences.”
The traceability issues with this untraceable horse/donkey meat also bears some similarity to the problem of kangaroo meat diverted into the human food chain in Australia. Kangaroo meat is often obtained from animals that were shot with machine guns via helicopters and therefore not slaughtered humanely and not bled by conventional standards either. Possibly also introduced into the food chain dubiously as well.
The ability to treat horses with bute is very important for their welfare. The EU scandal has also revealed that the passporting system there is subject to fraud, despite strict rules regarding the regulation of medicines. The fault lies not with horse owners but with individuals or organizations who are motivated by greed and willing to manipulate the system, allow their controls to fail, or commit outright fraud.
Food safety laws are clear. Companies that produce, trade or sell food or food ingredients are legally obligated to implement a quality assurance system called Hazard Analysis and Critical Control Point  (HACCP), which maximizes food safety by minimizing chemical, physical and microbiological hazards. There is something wrong with a food system whereby the food animal must sit on a feedlot for six months in order that veterinary drugs “degrade,” and 100% of the “raw material” must pass a negative test before they can enter the food chain.
(HACCP), which maximizes food safety by minimizing chemical, physical and microbiological hazards. There is something wrong with a food system whereby the food animal must sit on a feedlot for six months in order that veterinary drugs “degrade,” and 100% of the “raw material” must pass a negative test before they can enter the food chain.
In the final analysis, no one is really in a position to make broad statements about the safety of this horse/donkey meat. Conrad Brunk, the co-chair of the 2001 Royal Society of Canada Expert Panel on the Future of Food Biotechnology, wrote that:
“When it comes to human and environmental safety there should be clear evidence of the absence of risks; the mere absence of evidence is not enough.” This is the essence of the precautionary principle, which states that “when an activity raises threats of harm to human health or the environment, precautionary measures should be taken even if some cause-and-effect relationships are not fully established scientifically.”

